
(CHEM 1407) Solubility Rules Worksheet Answers
Solubility Rules Worksheet. Name or give the chemical formula for each of the following compounds. State whether they are soluble (will dissolve) or insoluble (will not dissolve) in solution. Use solubility rules. Chemical Formula. Name. Solubility. 1. NH4CH3COO.

Solubility Rules Worksheet Pdf worksheet
10. Periodic Properties of the Elements 1h 47m. The Electron Configuration 10m. The Electron Configuration: Condensed 2m. The Electron Configurations: Exceptions 9m. The Electron Configuration: Ions 12m. Paramagnetism and Diamagnetism 4m. The Electron Configuration: Quantum Numbers 7m. Valence Electrons of Elements 8m.
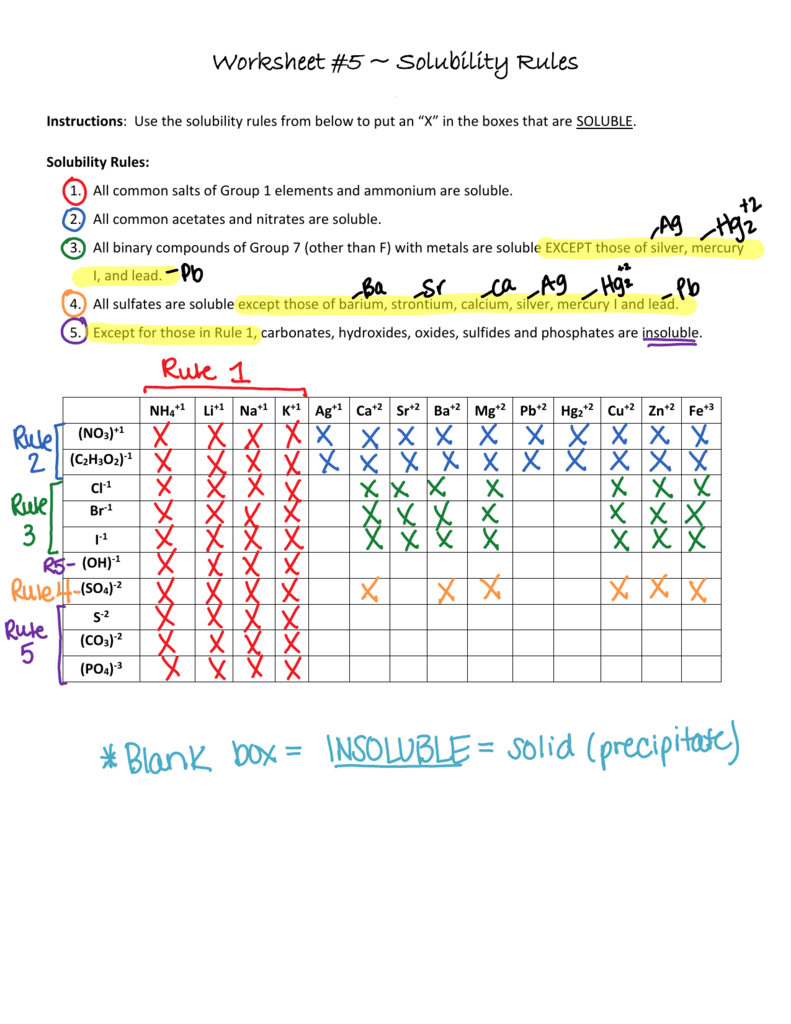
Solubility Rules Worksheet KEY
How soluble is it? Not all soluble substances dissolve equally. In this science worksheet, your child learns about differing rates of solubility by reading a bar chart and interpreting the data to answer questions. SCIENCE | GRADE: 5th. Print full size.

17 Who Rules Worksheet Answers /
Dunigan Science. This file is intended for honors, pre-ap, or AP chemistry. It includes teacher instructions, a student handout for 5 solubility rules, a student worksheet, and a key. Students have 40 problems. They determine if the ionic compound is soluble or insoluble using the rules, determine which rule (s) apply, and write the formula.

Solubility Rules Chart in Word and Pdf formats page 2 of 2
Calculate the solubility in moles/L of each of three salts and the concentration of the cations in mg/mL in each of the saturated solutions. AgCN A g C N with Ksp = 2.0 ×10‐12 K s p = 2.0 × 10 ‐ 12. BaSO4 B a S O 4 with Ksp = 1.5 ×10‐9 K s p = 1.5 × 10 ‐ 9. FeS F e S with Ksp = 3.7 ×10‐19 K s p = 3.7 × 10 ‐ 19.

1. Solubility Rules Practice Worksheet State whether the following
from 30 to 100. Slightly soluble. from 100 to 1,000. Very slightly soluble. from 1,000 to 10,000. Practically insoluble. more than 10,000. Review the solubility rules for common ionic compounds in water and download our solubility rules chart for easy reference. Look up compounds like calcium carbonate, barium sulfate, and sodium sulfate.

Solubility Rules Doodle Notes Doodle notes, Chemistry activities, Fun
Answers to Solubility Rules Worksheet. 3. Classify each of the substances as being soluble or insoluble in water. zinc carbonate - insol . 4. Identify the two new compounds which form if the solutions, as suggested by the following table, were mixed. CIRCLE the names of the compounds which would precipitate from the solutions.
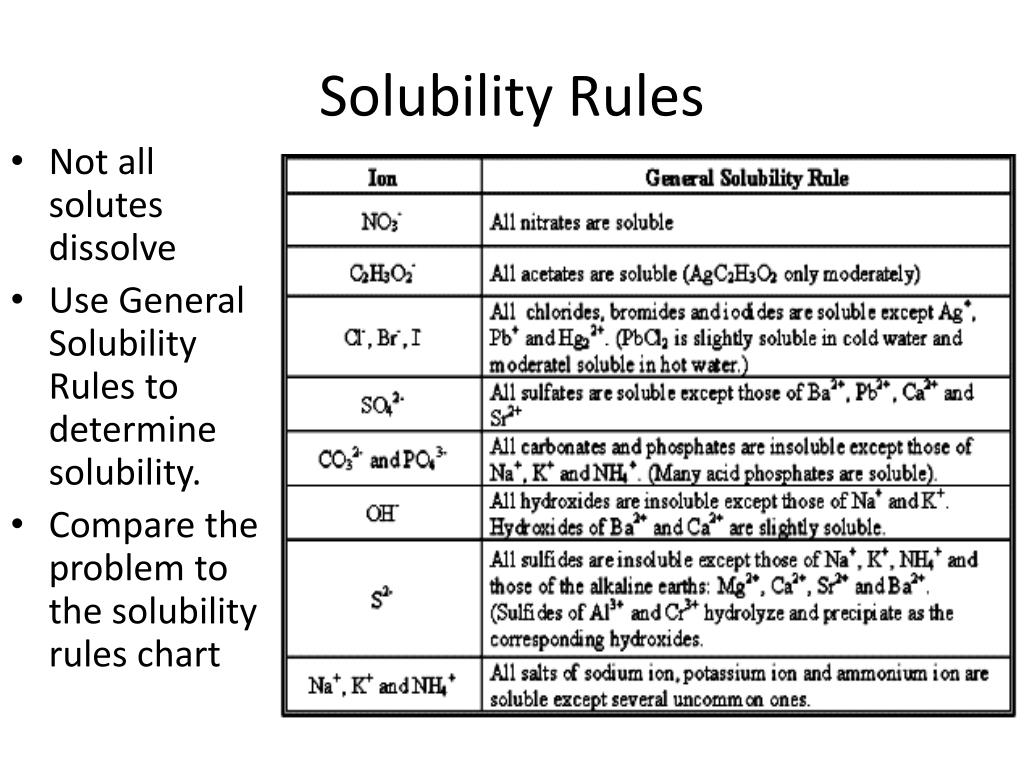
PPT Solubility Rules PowerPoint Presentation, free download ID5256674
Salts containing Cl -, Br -, or I - are generally soluble. Important exceptions to this rule are halide salts of Ag+, Pb2+, and (Hg2)2+. Thus, AgCl, PbBr2, and Hg2Cl2 are insoluble. Most silver salts are insoluble. AgNO3 and Ag (C2H3O2) are common soluble salts of silver; virtually all others are insoluble.

Unit VIB Solubility Rules Worksheet
12. Introduction to Organic Chemistry 1h 6m. 13. Alkenes, Alkynes, and Aromatic Compounds 1h 23m. 14. Compounds with Oxygen or Sulfur 31m. 15. Aldehydes and Ketones 37m. Learn Solubility Rules with free step-by-step video explanations and practice problems by experienced tutors.

19 Best Images of Importance Of Following Rules Worksheet Following
2AgNO 3 + Na 2 S → Ag 2 S + 2NaNO 3. A precipitate form if either Ag 2 S or NaNO 3 is insoluble. From the solubility rules, sulfides tend to be insoluble, so Ag 2 S likely forms a precipitate. NaNO 3 is soluble and does not form a precipitate because most nitrates are soluble. Since Ag 2 S forms a precipitate, one does form in this reaction.

Solubility Worksheet Answers worksheet
Liveworksheets transforms your traditional printable worksheets into self-correcting interactive exercises that the students can do online and send to the. Chemistry (2229004) Main content: Solubility rules (2229005) Loading ad. Share / Print Worksheet. Google Classroom Microsoft Teams Facebook Pinterest Twitter.

Use this worksheet for chemistry engagement! Students will enjoy
Calculate the solubility in moles/L of each of three salts and the concentration of the cations in mg/mL in each of the saturated solutions. AgCN. A g C N. with Ksp = 2.0 × 10 ‐ 12. K s p = 2.0 × 10 ‐ 12. BaSO4.

Solubility Rules Practice Worksheet worksheet
Solubility Rules Worksheet As you work through the steps in the lab procedures, record your experimental values and the results on this worksheet. Data Table A: Investigating Trends in Solubility NH 1+ 4 K Ca 2+Sr Mg Al 3+Fe Zn Cl1 ClO1 4 OH1 CO2 3 SO2 4 PO3 4 Additional Observations:
15 Solubility Rules Worksheet Answers /
Solubility Rules and Common Ions Solubility Rules NO3-1 All nitrates are soluble. Cl-1 All chlorides are soluble except: AgCl, PbCl2, and Hg2Cl2. SO4-2 All sulfates are soluble except: Ag2SO4, PbSO4, Hg2SO4, CaSO4, SrSO4, and BaSO4. CO3-2 The carbonate of Group 1 metals and (NH4)2CO3 are soluble. All other carbonates are insoluble. OH-1 The hydroxides of Group 1 metals, and the heavier Group 2

solubility rules practice worksheet Cindy Diagram
Solubility Rules Practice Worksheet 1. Name or give the chemical formula for each of the following compounds. 2. State whether they are soluble (will dissolve) or insoluble (will not dissolve) in solution. Use solubility rules. Chemical Formula Name Solubility 1. NH 4 C 2 H 3 O 2 2. Ba(OH) 2 3. Iron (II) Carbonate 4. NaOH
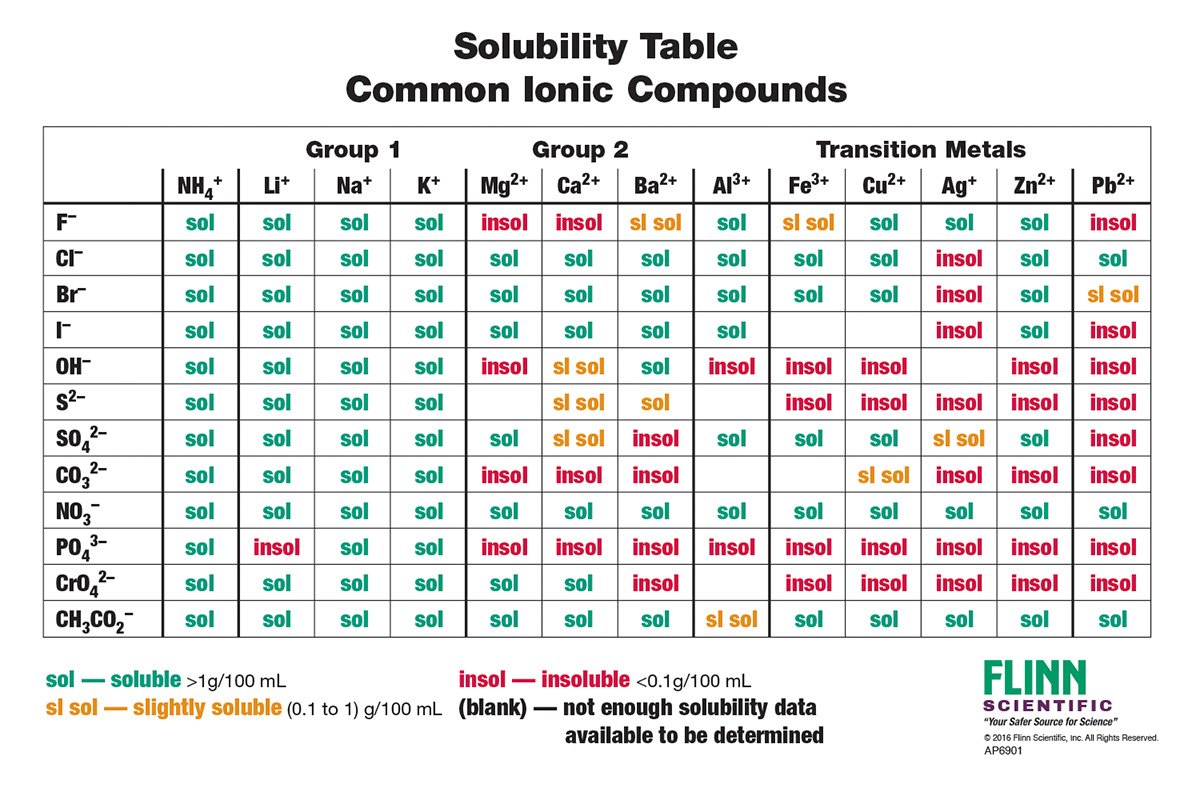
Solubility Rules Charts for Chemistry
Generally Soluble. Carbonates. Phosphates. Oxides. Sulfides. Generally insoluble. Except when with ammonium (NH4) Potassium (K) and Sodium (Na) Hydroxides. Insoluble.